Winter Park, Florida--(Newsfile Corp. - January 13, 2026) - ADIA Nutrition Inc. (OTCQB: ADIA), today announced that its subsidiary, Adia Med of Winter Park, LLC., has officially begun the recruitment process for a groundbreaking clinical study evaluating the potential benefits of stem cell therapy in children with Autism Spectrum Disorder (ASD).
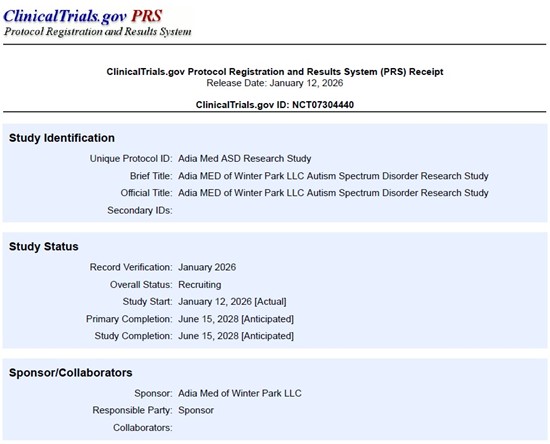
The 24-month randomized interventional study, officially titled "Adia MED of Winter Park LLC Autism Spectrum Disorder Research Study" and listed on ClinicalTrials.gov (NCT07304440), is being conducted at Adia Med's clinic in Winter Park, Florida.
Adia MED of Winter Park LLC Autism Spectrum Disorder Research Study: https://clinicaltrials.gov/study/NCT07304440
Adia Med ASD Study Recruiting Status
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/10520/280212_adiaasd.jpg
ClinicalTrials.gov has provided receipt confirmation that the study is now in recruiting status, and it should appear publicly in recruiting status on the site within the next few days.
The trial aims to enroll approximately 100 children ages 3-12 with a confirmed ASD diagnosis. This study investigates whether combining AdiaVita-Adia Med's proprietary umbilical cord blood-derived stem cell and exosome product-with glutathione therapy provides greater improvement in autism symptoms compared to glutathione therapy alone. Participants will be randomly assigned to one of two groups for the initial three-month phase:
- One group will receive glutathione only.
- The other will receive glutathione plus monthly intravenous infusions of AdiaVita.
Success will be assessed over the full 24 months using the Autism Treatment Evaluation Checklist (ATEC), completed by parents as well as therapists or teachers. The primary outcome is the change in total ATEC scores at six months, with additional focus on safety, tolerability, quality of life, and overall well-being through regular clinic visits, physical exams, blood tests, and adverse event monitoring. After the initial phase, children in the glutathione-only group will have the opportunity to cross over and receive AdiaVita infusions at no additional cost.
In the last year of its operations, Adia Med of Winter Park has observed tremendous results from its one-off studies with children that have ASD.
"Launching recruitment for this important study marks a significant milestone in our commitment to advancing regenerative therapies for autism," said Larry Powalisz, CEO of Adia Nutrition Inc. "We are excited to explore the potential of AdiaVita combined with glutathione to support families affected by ASD and contribute meaningful data to the scientific community."
Participation Details and Costs
Participation in the study is completely voluntary, and families may withdraw at any time without penalty (though fees already paid for completed procedures are non-refundable). There is a one-time fixed participation fee of $12,000 that covers:
- All treatments and procedures during the initial three-month Phase One (regardless of which group the child is randomized to)
- All required clinic visits, monitoring, physical exams, blood tests, and safety assessments throughout the full 24-month study
- Entry into the optional Phase II crossover extension (including AdiaVita infusions) at no additional cost for families initially assigned to the glutathione-only group.
No monetary incentives or compensation are provided to participants. Interested families are encouraged to contact Adia Med directly for eligibility screening, a full breakdown of what the fee covers, payment options, and additional information.
Clinic owners and healthcare practitioners interested in licensing the Adia Med name or integrating Adia's regenerative therapies into their practice are encouraged to reach out directly. Strategic partnerships are welcomed as part of Adia's continued mission to expand access to advanced stem cell solutions.
About ADIA Nutrition Inc.:
Adia Nutrition Inc. (OTCQB: ADIA), based in Winter Park, Florida, is a publicly traded company advancing healthcare through innovation. The company specializes in sales of stem cell and regenerative products, such as AdiaVita and AdiaLink, through its lab division, Adia Labs LLC, which is expanding to include insurance-billable wound care products. Adia is also growing nationwide with Adia Med clinics, specializing in orthopedic, pain management, and wound repair. Adia Med clinics also offer specialized regenerative treatments like stem cell therapies and platelet-rich plasma (PRP), advanced treatments including therapeutic plasma exchange (TPE) and autologous hematopoietic stem cell transplantation (aHSCT), and wound repair services.
Revenue is generated through service fees, product sales, equity stakes, and billing insurance for healthcare treatments. Additionally, Adia Nutrition Inc. invests in aligned businesses such as Cement Factory LLC, a nutrition and supplement company with shared values and a focus on health and wellness. Through bold partnerships with top-tier medical entities and unwavering dedication to standardized, FDA-approved lab protocols, Adia Nutrition Inc. is revolutionizing healthcare, igniting a nationwide movement to empower communities with groundbreaking regenerative solutions and vibrant, holistic wellness.
Website: www.adianutrition.com
Website: www.adiamed.com
Website: www.adialabs.com
Website: www.cementfactory.co
Twitter (X): @ADIA_Nutrition
Safe Harbor: This Press Release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements are based on the current plans and expectations of management and are subject to a few uncertainties and risks that could significantly affect the company's current plans and expectations, as well as future results of operations and financial condition. A more extensive listing of risks and factors that may affect the company's business prospects and cause actual results to differ materially from those described in the forward-looking statements can be found in the reports and other documents filed by the company with the Securities and Exchange Commission and OTC Markets, Inc. OTC Disclosure and News Service. The company undertakes no obligation to publicly update or revise any forward-looking statements, because of new information, future events or otherwise.

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/280212

© 2026 Canjex Publishing Ltd. All rights reserved.